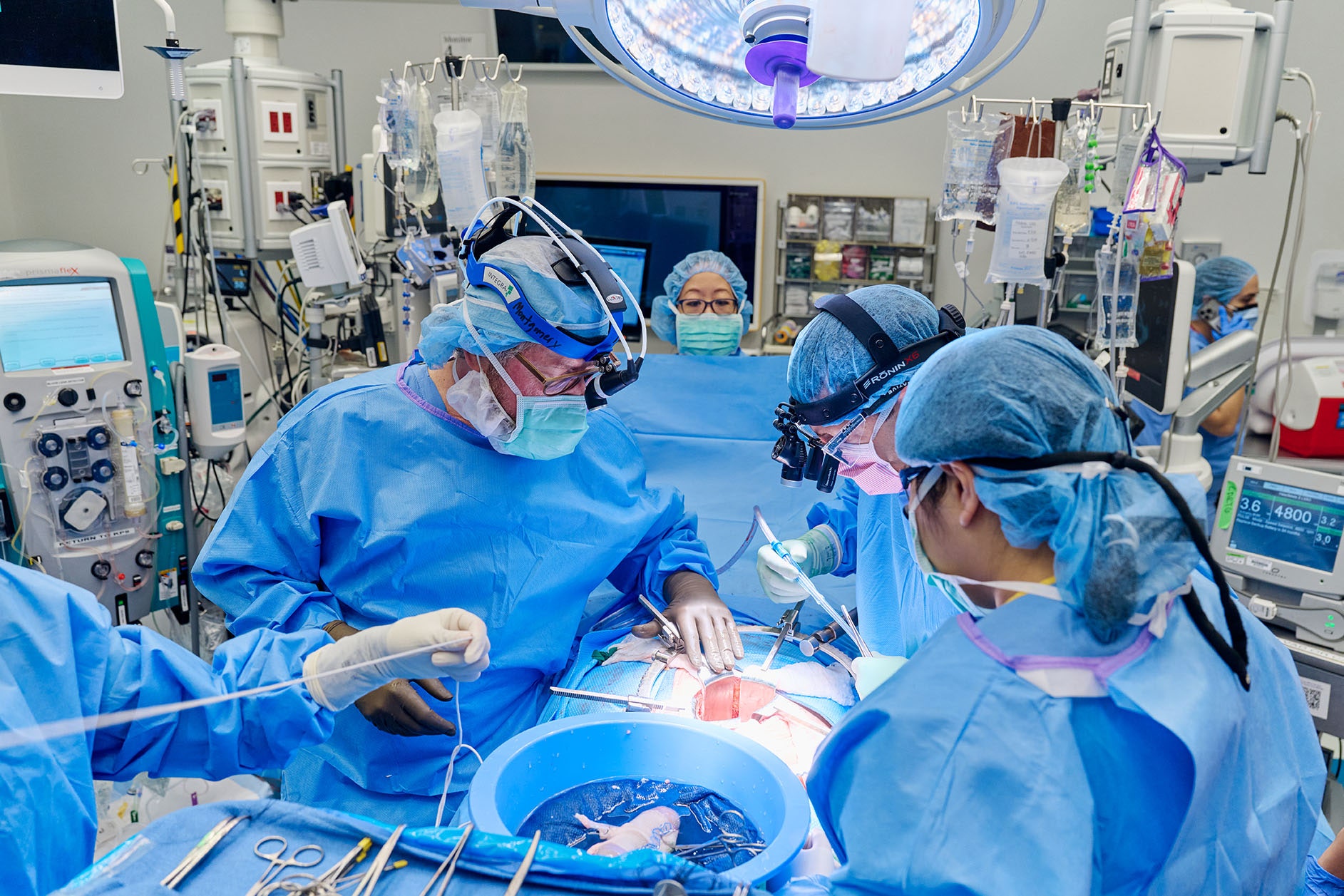
Surgeons in New York have removed a pig kidney less than two months after transplanting it into Lisa Pisano, a 54-year-old woman with kidney failure who also needed a mechanical heart pump. The team behind the transplant says there were problems with the heart pump, not the pig kidney, and that the patient is in stable condition.
Pisano was facing heart and kidney failure and required routine dialysis. She wasn’t eligible to receive a traditional heart and kidney transplant from a human donor because of several chronic medical conditions that reduced the likelihood of a good outcome.
Pisano first received a heart pump at NYU Langone Health on April 4, followed by the pig kidney transplant on April 12. The heart pump, a device called a left ventricular assist device or LVAD, is used in patients who are either awaiting heart transplantation or otherwise aren’t a candidate for a heart transplant.
In a statement provided to WIRED, Pisano’s medical team explained that they electively removed the pig kidney on May 29—47 days after transplant—after several episodes of the heart pump not being able to pass enough blood through the transplanted kidney. Steady blood flow is important so that the kidney can produce urine and filter waste. Without it, Pisano’s kidney function began to decline.
“On balance, the kidney was no longer contributing enough to justify continuing the immunosuppression regimen,” said Robert Montgomery, director of the NYU Langone Transplant Institute, in the statement. Like traditional transplant patients, Pisano needed to take immunosuppressive drugs to prevent her immune system from rejecting the donor organ.
The kidney came from a pig genetically engineered by Virginia biotech company Revivicor to lack a gene responsible for the production of a sugar known as alpha-gal. In previous studies at NYU Langone, researchers found that removing this sugar prevented immediate rejection of the organ when transplanted into brain-dead patients. During Pisano’s surgery, the donor pig’s thymus gland, which is responsible for “educating” the immune system, was also transplanted to reduce the likelihood of rejection.
A recent biopsy did not show signs of rejection, but Pisano’s kidney was injured due to a lack of blood flow, according to the statement. The team plans to study the explanted pig kidney to learn more.
Pisano is now back on dialysis, a treatment for kidney-failure patients, and her heart pump is still functioning. She would not have been a candidate for the heart pump if she had not received the pig kidney.
“We are hoping to get Lisa back home to her family soon,” Montgomery said, calling Pisano a “pioneer and a hero in the effort to create a sustainable option for people waiting for an organ transplant.”
Pisano was the second living person to receive a kidney from a genetically engineered pig. The first, Richard Slayman of Massachusetts, died in May just two months after the historic transplant. The surgery was carried out on March 16 at Massachusetts General Hospital. In a statement released on May 11, the hospital said it had “no indication” that Slayman’s death was the result of the pig kidney transplant. The donor pig used in Slayman’s procedure had a total of 69 different genetic edits.
The global donor organ shortage has led researchers including the NYU and Massachusetts teams to pursue the possibility of using pigs as an alternative source. But the body immediately recognizes pig tissue as foreign, so scientists are using gene editing in an effort to make pig organs look more like human ones to the immune system. Just how many gene edits will be needed to keep pig organs working in people is a topic of much debate.
Pig heart transplants have also been carried out in two individuals—one in 2022 and the other in 2023—at the University of Maryland. In both cases, the patients were not eligible for human ones. Those donor pigs had 10 genetic edits and were also bred by Revivcor. Both recipients died around two months after their transplants.